TECHNOLOGY
CRYOIMMUNOSTAINING™
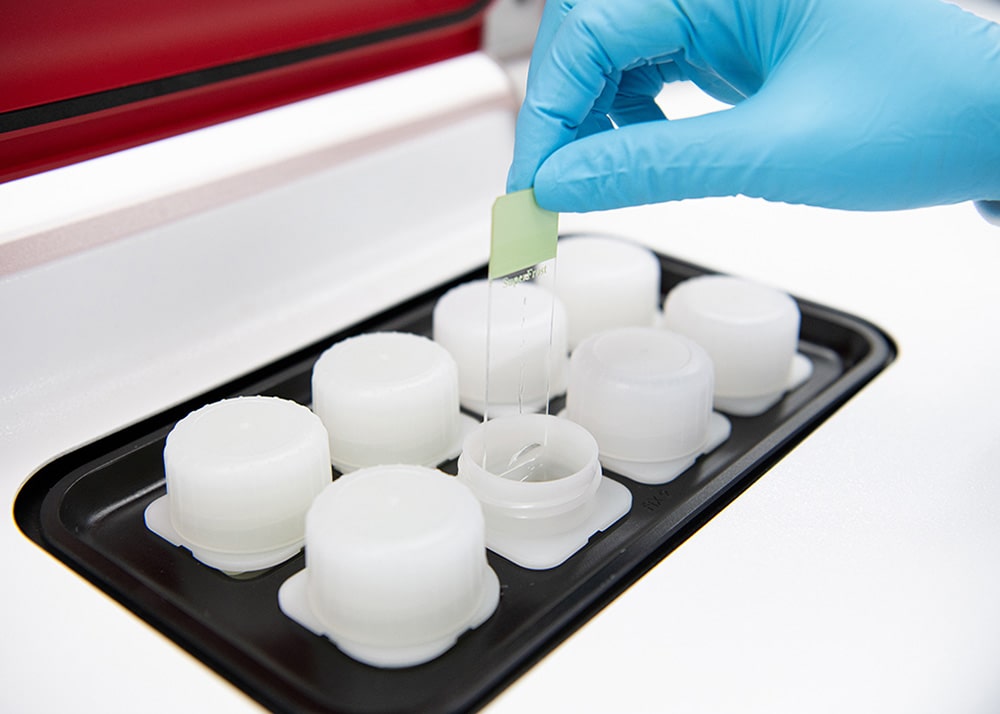
The patented X-ZELL Cryoimmunostaining™ Suite is a two-instrument slide staining system optimised for liquid samples such as bronchoalveolar lavage (BAL), pleural effusion (PE), pre-treated whole blood samples and ascites, as well as fine-needle aspirates (FNA).
Designed to slot seamlessly into routine cytology, both instruments operate at sub-zero temperatures to protect sample integrity and use patented buffers and reagents developed by X-ZELL.
- \No cytoblocking required
- \Only 1 slide per sample
- \Less than 4h turnaround time [1]


CRYOFIXATOR™

CRYOSTAINER™
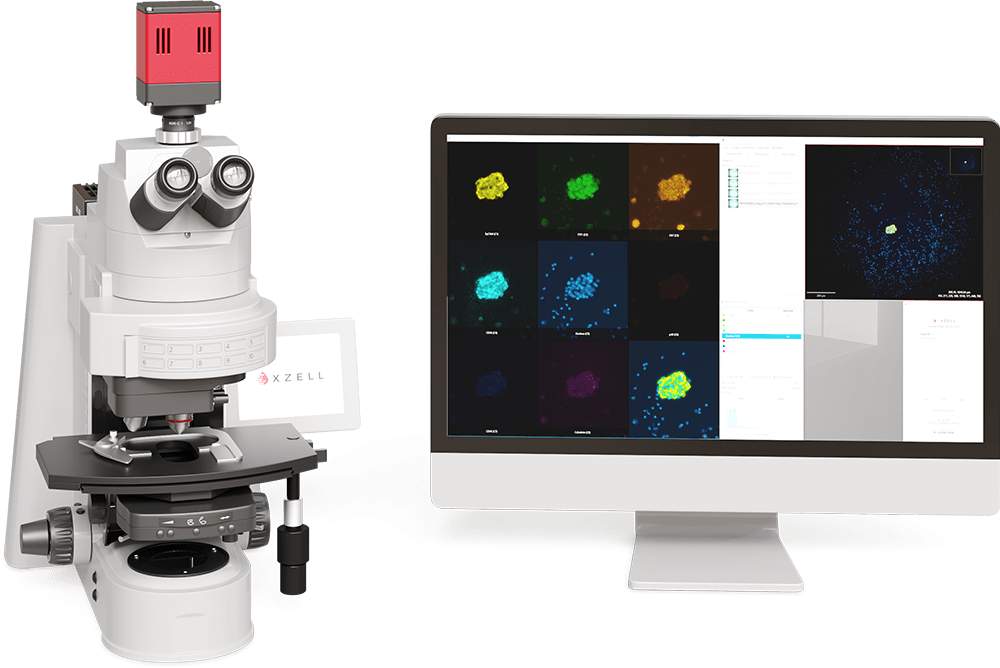
HYBRID MICROSCOPY
The X-ZELL Hybrid Microscope is a proprietary workstation allowing you to evaluate multiple fluorescence channels side-by-side in any lab or office or office environment. Simply pre-select an area of interest through the ocular lenses or live on screen and digitise the section for a new on-screen viewing experience.
- \See all antibody expressions sideby- side in a split-screen format
- \Browse the slide section in real time
- \Compare conspicuous cells live on screen
- \Switch each channel on/off and combine as required

hMX ™ CELL SEPARATION
What if we took body fluid analyses to the next level? To answer that question, X-ZELL developed the patented hMX™ cell separation system, which gently removes leucocytes from whole blood to expose single atypical cells and ready them for downstream Cryoimmunostaining™. The hMX™ system is currently part of a revolutionary dual-centre trial in Singapore, where it will pave the way for a new age of affordable, widely accessible cancer diagnostics. More here.
X-ZELL laboratory instruments have been validated for use in combination with official X-ZELL consumable and reagent kits. Third-party reagents and consumables have not been validated and may affect instrument performance. The X-ZELL Cryofixator™ and Cryostainer™ are classified as products for general purpose laboratory use (US FDA Class 1 and CE-IVDR Class A). The X-ZELL Hybrid Microscope software is classified as low risk Class I SaMD (Software as a Medical Device) as defined by EU MDR 2017/745, Article 2. Unless specifically indicated, all other X-ZELL products are designated as general laboratory equipment.
¹ X-ZELL data. More information upon request
© 2024 X-ZELL Biotech Pte. Ltd.
Find Us